42 eu language requirements for product labels
United States Product Labeling Requirements: An Overview - Compliance Gate The label should be conspicuous, whether it is on the product or its packaging. The care label covers instructions may concern the following: Washing (e.g., "Machine wash") Drying (e.g., "Tumble dry") Ironing (e.g., "Iron") Bleaching (e.g., "Only non-chlorine bleach") Warning (e.g., "Wash with like colours") EU product requirements | European Commission Requirements for the use of chemicals (REACH) How to comply with the EU's classification, labelling and packaging regulation, how to classify chemicals, submitting a substance to the classification and labelling inventory. Classification, labelling, packaging of chemicals Details of chemical products that require additional or specific legislation.
Medical Device Labeling in the European Union | mddionline.com All medical devices and their accessories that have been placed on the European Union (EU) market since June 15, 1998, have had to comply with the essential requirements set forth by the Medical Devices Directive, including the CE mark and labeling requirements. Directive 93/42/EEC was passed on June 14, 1993, with a five-year transition period ...

Eu language requirements for product labels
Product Labeling Requirements: What You Need To Know The US, Canada, Mexico, and the EU all require that your product packaging be written in local languages. In Canada, that means your labels need to be in English and French. In Mexico, that means Spanish. Choosing a Labeling Translation and Compliance Partner Country Language 1 Language 2 Language 3 - Europa 1) Please check with the local authorities, requirements can vary depending on region. 2) German OR French 3) The labelling must be written in at least two official languages. With the agreement of individual professional final users, a substance for supply to these final users may be labelled in only one official language or in English. EU labels | European Commission EU Ecolabel- Products covered by the EU's Ecolabel initiative, criteria for establishing an Ecolabel, how to apply for an Ecolabel, application and annual fee rates. Energy labels. Energy efficient products - Requirements for energy efficient products, EU energy labelling and ecodesign rules, the EU's energy star programme.
Eu language requirements for product labels. Official Language in EU required for Medical Device Labeling User interface Language Requirements - EU MDR: EU Medical Device Regulations: 1: Nov 6, 2020: C: Non-EU Language Requirements: Other Medical Device Regulations World-Wide: 3: Aug 10, 2020: K: China Medical Device Labeling requirements - Language: China Medical Device Regulations: 9: Jun 15, 2020: The unbearable insensitivity of risk management ... EU - Labelling Requirements | CE Intelligence The use of language on labels has been the subject of a Commission Communication, which points out that labelling of foodstuffs for sale to the final consumer must be in an easily understandable language which is generally interpreted to mean the language of the country of marketing (European Commission ,2010). European language labeling for Medical Devices CE Mark EU Foreign Language Labeling Requirements. There are two major areas of confusion about the translation requirements when CE marking a product for export to the EU States. One is that everyone talks about "CE Marking translation requirements" without actually reading the specific Directive(s) that apply to their products. EU Language Requirements | Obelis EU Language Requirements EU Language Requirements The label of a cosmetic product (container & outer packaging) should follow the language requirements which are exerted by the National Laws of the EU member states.
Product-information requirements | European Medicines Agency EMA's guidance explains the content that should be included in these documents, as well as standard headings and the most commonly used standard statements and terms in all official European Union (EU) languages plus Icelandic and Norwegian, and defines the format and layout for the product information. EMA's guidance is without prejudice to: Switzerland - Labeling and Marking Requirements Generally, labeling and marking requirements follow EU regulations (CE labeling); however, a CE mark is not required for a product made only for Swiss domestic use. SECO coordinates the implementation of the Federal Act on Product Safety and more information can be found on their webpage on product safety (available in German, French, and Italian). EU MDR language requirements — what manufacturers and ... - Decomplix In the explanations on technical documentation in Annex II, there is also a list of the information that the manufacturer must include in the respective official languages, namely the label (s) on the product and its packaging (single unit packaging, sales packaging, transport packaging) as well as the instructions for use. GUI for medical software Product Labeling Regulations in the US, EU and Australia Here are few labeling requirements for imported products: a. The label must contain the identity of the prepackaged product in terms of its function or generic name. b. The label must contain the principal place of business and identity of the manufacturer or dealer. c.
EU countries: general product requirements | Business.gov.nl Language requirements For many product categories, the EU has legal language requirements for product labels, packaging markings, technical instructions, manufacturers' declarations, and user instructions. These language requirements are often part of EU health and safety measures for specific product groups (such as CE marking and REACH/CLP). How to Create a Label as per EU MDR 2017/745? Innovations by MDR 2017/745. Manufacturers of medical devices are subject to a number of new requirements as a result of the EU Medical Device Directive, mainly the following: The scope of application also extends to non-medical products (e.g. contact lenses, devices for liposuction). Each medical device must bear a unique identification number ... A Brief Reminder of the Language Requirements - Biorius Minimal legal requirements imposed by the European Cosmetics Regulation. According to Article 19 §5 of the EU Cosmetics Regulation, distributors have to ensure that a certain number of labeling requirements are properly translated in the national language(s) of the countries where the products are intended to be sold. These labeling ... EU Cosmetic Labeling Requirements | CosmeReg What are the requirements for EU cosmetic labeling? To successfully place your cosmetic product on the EU, read our article! Kakaotalk ID: Cosmereg +44 20 33182439 +1 727 3509380; ... You must translate the function of the product into the native language of each country the product is being introduced to.
European Union - Labeling/Marking Requirements (part 1) EU - export Summary. There is a broad array of EU legislation pertaining to the marking, labeling and packaging of products, with neither an "umbrella" law covering all goods nor any central directory containing information on marking, labeling and packaging requirements.
Labels and markings - Your Europe Most footwear sold in the EU must bear a label that informs potential buyers what they are made of. Footwear Label Clothes and other textile products sold in the EU are required to carry a label with information on the textile fibre composition. This enables your customers to make an informed decision when they buy. Textile Label
Labelling and packaging | Access2Markets - Europa This page serves as a reference document only for EU-wide product requirements. Extra requirements may apply depending on the destination EU country. Please refer to My Trade Assistant for full details. Please be aware that in this page, general description of each heading is provided in all EU languages. However, the details are only available ...
Product Labelling — EUbusiness.com | EU news, business and politics A summary of the EU product labelling rules for goods sold within the Single Market. The purpose of product labelling is to provide complete information on the content and composition of products in order to protect the consumer's health and interest. In turn the incentive for manufacturers is that a well labelled product significantly ...
European Language Translation Requirements for Medical Device Labeling ... An * indicates that that English may be acceptable for devices used only by healthcare professionals. Even so it is always recommended to translate into an official language of that country. AUSTRIA - German BELGIUM - Dutch, French and German BULGARIA - Bulgarian* CROATIA - Croatian CYPRUS - Greek* CZECH REPUBLIC - Czech DENMARK - Danish
EU: Language Requirements for Product Labels - GlobalTrade.net Belgium has three official languages: Dutch, French and German. All information which is required to appear on labels must be in the official language of the region where the product is commercialized (if necessary, further requirements for specific products may be obtained from the importer).
European Union Product Labelling Requirements: A Complete Guide EU Textiles Labelling Clothing and other products containing a minimum of 80% by weight of textile fibres must be labelled with the correct fibre composition (e.g. 100% Cotton or 100% Polyester). Further, the label must be permanent, which means it must either be attached to the clothing item or printed. A sticker is not enough. Product Examples
EU Labeling Requirements - United States Mission to the European Union Nutrition labeling becomes mandatory on December 13, 2016 Minimum font size for printing mandatory information New format for allergen labeling (allergens must be highlighted in the list of ingredients - "allergen boxes" are no longer allowed) Voluntary front-of-pack labeling has to follow a set format
Textile Label - Your Europe Every textile product must be labelled or marked to show its fibre composition whenever the product is marketed in the EU. These labels must be firmly attached to the product, for example, sewn in. This requirement concerns all products made up of at least 80% of textile fibres, calculated by weight, such as: clothing furniture coverings
EU - Labeling/Marking Requirements - International Trade Administration Effective July 16, 2021, the EU required all CE marked products to have a label that identified a point of contact within the region. This requirement applies to products sold online and through traditional distribution channels.
EU labels | European Commission EU Ecolabel- Products covered by the EU's Ecolabel initiative, criteria for establishing an Ecolabel, how to apply for an Ecolabel, application and annual fee rates. Energy labels. Energy efficient products - Requirements for energy efficient products, EU energy labelling and ecodesign rules, the EU's energy star programme.
Country Language 1 Language 2 Language 3 - Europa 1) Please check with the local authorities, requirements can vary depending on region. 2) German OR French 3) The labelling must be written in at least two official languages. With the agreement of individual professional final users, a substance for supply to these final users may be labelled in only one official language or in English.
Product Labeling Requirements: What You Need To Know The US, Canada, Mexico, and the EU all require that your product packaging be written in local languages. In Canada, that means your labels need to be in English and French. In Mexico, that means Spanish. Choosing a Labeling Translation and Compliance Partner


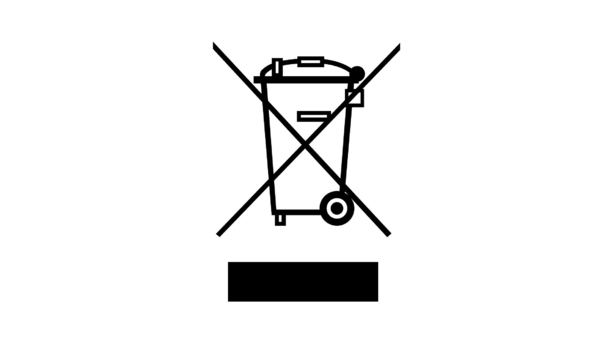





















Post a Comment for "42 eu language requirements for product labels"